Home Top Stories Local facilities being retrofitted to store COVID-19 vaccines – Health Ministry
…as 30 new cases recorded
Guyana has reported an increase of 30 new cases of the novel coronavirus (COVID-19), which raised the overall positive figures to 5406. Of this amount, however, 4392 patients have completely recovered.
Locally, 2786 males and 2620 females have contracted the virus ever since it was first detected here on March 11.
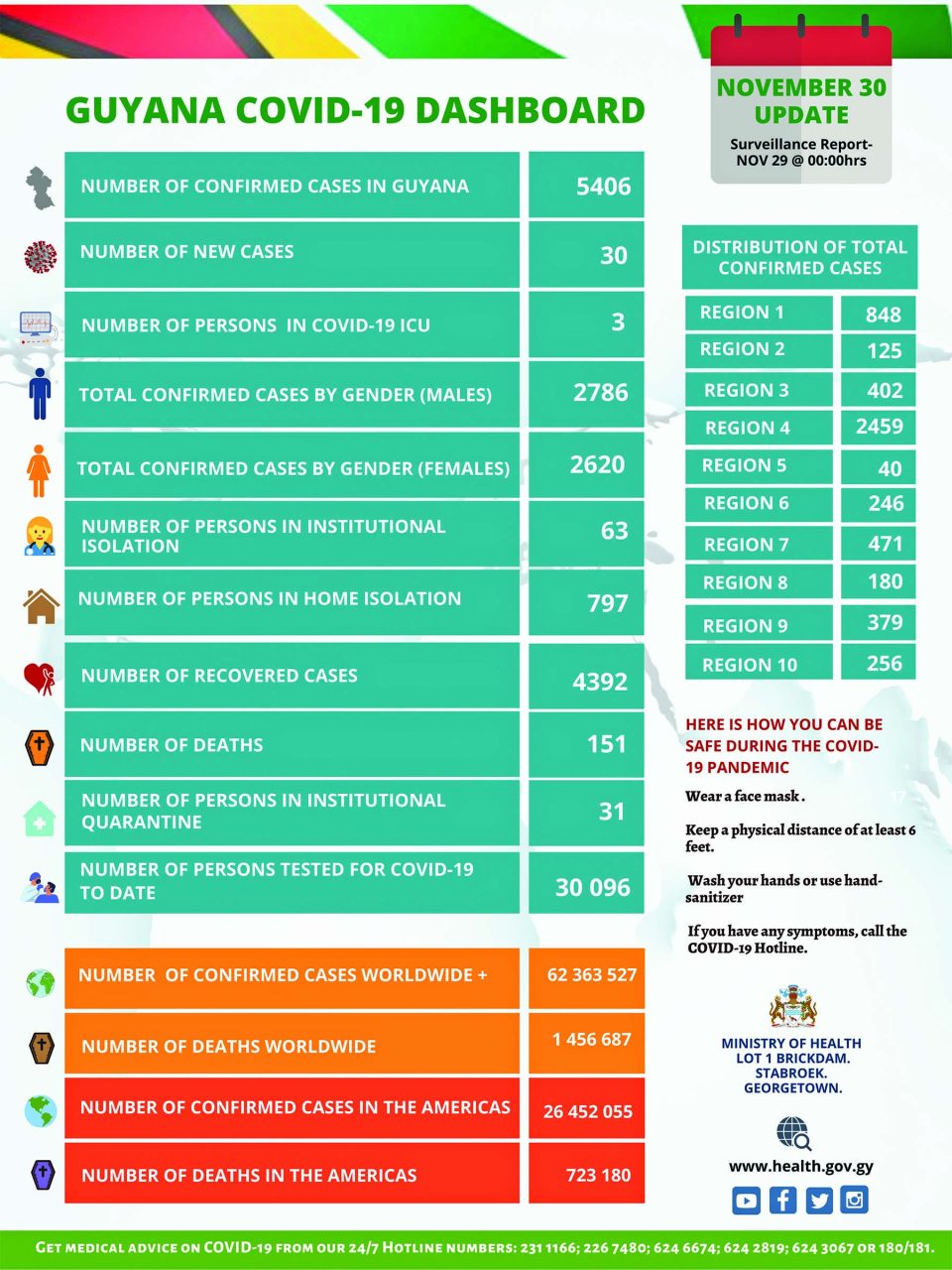 With 151 deaths, there are three persons in the Intensive Care Unit, 63 persons in institutional isolation, 797 in home isolation and 31 in institutional quarantine.
With 151 deaths, there are three persons in the Intensive Care Unit, 63 persons in institutional isolation, 797 in home isolation and 31 in institutional quarantine.
A breakdown showed that two new cases were detected in Region Two (Pomeroon-Supenaam), four in Region Three (Essequibo Islands-West Demerara), two in Region Four (Demerara-Mahaica), 16 in Region Six (East Berbice-Corentyne), five in Region Seven (Cuyuni-Mazaruni) and one in Region 10 (Upper Demerara-Berbice).
The cases in Regions One (Barima-Waini), Five (Demerara-Mahaica), Eight (Potaro-Siparuni) and Nine (Upper Takutu-Upper Essequibo) remain at 848; 40; 180 and 379 respectively.
Orealla and Siparuta villages in Region Six had recently experienced a surge in COVID-19 cases. Health Minister, Dr Frank Anthony said there are approximately 49 cases in Siparuta and 31 in Orealla.
A medical team is on the ground. He pointed out that before the active cases drop, there will be a slight increase in the area. Lockdown and imposed travel restrictions continue until there is some positive indication that persons are no longer at risk.
Health Minister, Dr Frank Anthony on Monday disclosed that the Intensive Care Unit of the Infectious Diseases Hospital at Liliendaal, East Coast Demerara, is finally operationalised, and patients desiring such medical care have since been transferred.
These patients were transferred from GPHC to the new facility – which has a capacity for 29 severely-ill persons.
“As of Friday, what we’ve been able to do is transfer all the ICU patients that we have at GPHC over to the Ocean View facility. We have the capacity there that is much larger than GPHC. GPHC was catering for about 15 patients. At Ocean View, we have capacity for 25 patients,” Dr Anthony shared.
It has been some weeks since the Ministry was working to fully operationalise the ICU facility. While there were some delays in the past, all equipment and instalments are completed.
“It took us a while to get the ICU up and running but all the things that were necessary for an ICU are now in place and we currently have at Ocean View in the Infectious Diseases ICU, 14 patients.”
Vaccine storage
As companies move closer to start producing a vaccine for COVID-19, the Health Ministry is also working to get all storage facilities in order, in preparation for the vaccine distribution.
Dr Anthony shared that a team has been dispatched to inspect bonds, and where necessary, upgrades will be made.
“We have regional storage facilities for vaccines and we have been doing an assessment of the regional storage facilities that we have. We’ll be upgrading all of them to ensure that they have the capacity to be able to manage a COVID-19 vaccine,” he shared.
Along with the expansion, freezers and power units will be added to ensure that the vaccines are kept at the designated temperature.
“Within the next few weeks, all these facilities will be upgraded, expanded in some cases, because we need additional space and we will be adding new freezers, appropriate for the type of vaccines that we will be getting. In some cases, we will have to do some power upgrades. We’ll have to keep these vaccines at a certain temperature and if in some cases we need backup generators, then we need to put those in place as well,” Dr Anthony said.
Last week, AstraZeneca revealed that its COVID-19 vaccine is around 90 per cent effective, cheaper to produce and there are no issues with distribution. Moreover, 700 million doses could be produced in the first quarter of 2021.
This comes after Pfizer and Moderna would have achieved 95 per cent effectiveness in the clinical trials of their vaccines. They have applied for emergency authorisation use from the US Food and Drug Administration, and this permission can give the greenlight for December production. With these vaccines, cold chain storage is required.
AstraZeneca’s vaccine does not require a cold chain, making it easier to use with the existing infrastructure. The Minister had indicated last week that while it is “promising”, authorities are awaiting more information from the detailed studies. Overall, the number of potential vaccines under various trials present many options. (G12)
 With 151 deaths, there are three persons in the Intensive Care Unit, 63 persons in institutional isolation, 797 in home isolation and 31 in institutional quarantine.
With 151 deaths, there are three persons in the Intensive Care Unit, 63 persons in institutional isolation, 797 in home isolation and 31 in institutional quarantine.